編者按
如果從1963年Baruch S. Blumberg教授發現HBsAg算起,人類對抗HBV的歷史已經有半個多世紀。在第28屆亞太肝病學會年會(APASL2019)的特邀「State-of-the-Art」演講環節,中國香港天下仁心醫療集團主席廖家傑教授回顧了這場沒有硝煙但人類必須獲得勝利的戰爭。我們取得了哪些階段性的成績?勝利的曙光在何方?一起來從演講的精彩內容中獲得啟示。


廖家傑教授
作為一位在亞太地區執業三十多年的肝病專家,臨床中最令人痛苦的是,臨床上有很多患者沒有得到及時診斷和治療,就診時已處於肝病晚期、甚至是終末期肝癌(HCC),他們中間有很多在中年時就因肝病不幸離世。


此外,慢性HBV感染也是終末期肝硬化及其併發症的罪魁禍首,並且乙型肝炎再激活——無論是自發性的,還是由於使用免疫抑製劑治療誘發的,都可能導致暴發性肝衰竭。因此,從臨床角度來看,治療慢性乙型肝炎的終點應該是減少HBV感染相關的發病率和死亡率,即減少肝細胞癌、終末期肝硬化以及由於乙型肝炎再激活所導致的暴發性肝衰竭的發生(APASL慢性乙型肝炎治療指南,2015)。
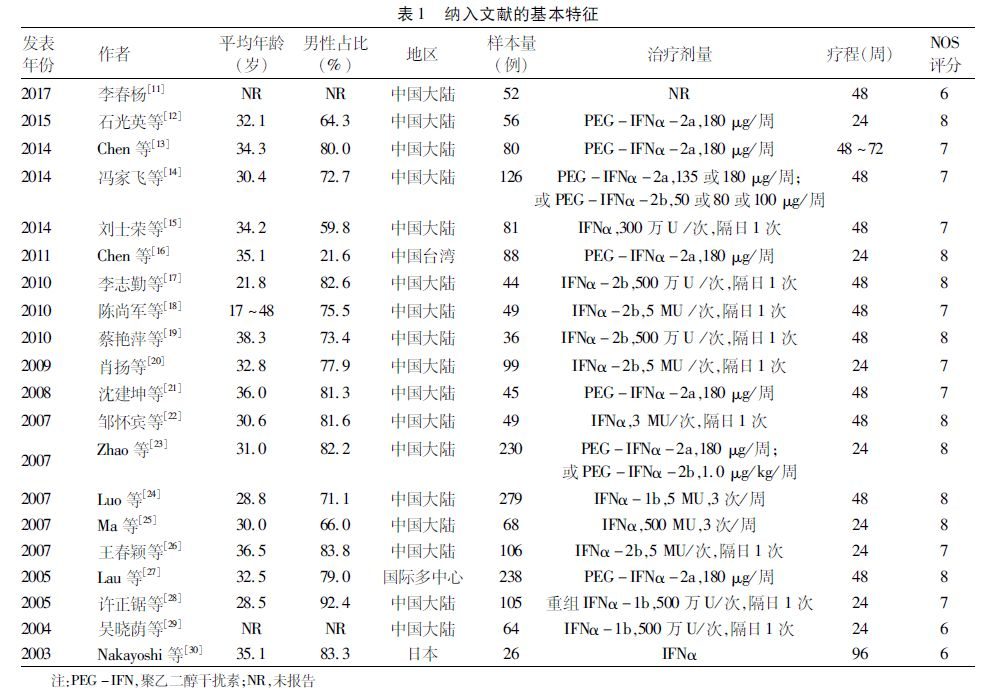
隨著對病毒生命周期的理解和對HBV感染中宿主免疫的重要性的認識,兩種干擾素(普通干擾素和聚乙二醇化干擾素)和7種核苷(酸)類似物:拉米夫定、替比夫定、恩替卡韋、克拉夫定(韓國獲批),阿德福韋酯、富馬酸替諾福韋二吡呋酯以及富馬酸丙酚替諾福韋,已在全球多個國家和地區獲批用於治療慢性乙型肝炎。


這些藥物的獲批是基於精心設計的隨機對照臨床試驗。這些試驗使用了替代治療終點(surrogate marker),如:HBeAg血清學轉換、血清HBV DNA水準降低、HBsAg轉陰和/或HBsAg血清學轉換(出現抗-HBs)和肝臟硬度改善。達到這些替代治療終點被認為「等同於」HBV相關肝病發病率和死亡率降低。
與此一致,一項長期的、精心設計的隨機對照研究證明,拉米夫定長期治療能夠有效地降低肝病相關的發病率和死亡率(Liaw YF,NEJM,2004[5])。然而,這二十餘年的臨床實際情況是,HBV相關的發病率和死亡率並未如預期下降。近期的系統性回顧研究(Anna Lok,Hepatology,2016[6])分析了73項研究,其中59項(15項隨機對照臨床試驗和44項觀察性研究)報導了臨床結果,僅有中等質量級別的證據支持「慢性乙型肝炎患者接受抗病毒治療能減少肝硬化、失代償期肝病以及HCC」。2018年,肝癌(至少80%是HCC)仍然是全球癌症死亡的第四大原因,全球有841,000例新發病例和782,000例死亡病例(死亡率/發病率為0.93)。每年,超過50%的新診斷肝癌病例(90%以上與HBV感染相關)和肝癌死亡病例發生在中國。另一方面,由於免疫抑製劑在腫瘤性疾病、免疫相關疾病和器官移植等患者人群中的應用增加,與HBV再激活相關的暴發性肝衰竭的發生率也在升高。
科學和現實臨床之間的聯繫,缺少了什麼?最近,主要的研究工作集中在實現B肝「治癒」——HBsAg轉陰伴或不伴出現抗-HBs以及清除肝內cccDNA。這是基於前期的觀察發現:在慢性乙型肝炎患者進展至肝硬化之前實現HBsAg轉陰,將大大降低HCC的發病率。


從實現消除HBV相關的肝臟發病率和死亡率的終點出發,這種類型的「治癒」在有效率、安全性和成本方面(隨著價格低廉的仿製葯廣泛使用),與長期乃至終生核苷(酸)類似物治療或有限療程的干擾素治療相比,是否會是更好的解決方案?
我們真正需要的是,開發基於基因組學和蛋白質組學的技術平台(Jiang Y,Nature,2019[7]),實現肝癌的早期診斷(從而使局部根治或者移植成為可能);提高社會公眾和醫療專業人員對慢性乙型肝炎的認識;設計更加行之有效的方案來進一步減少HBV的新發感染;以及剖析可能使HBV相關肝病發病率和死亡率升高的原因並加以糾正。
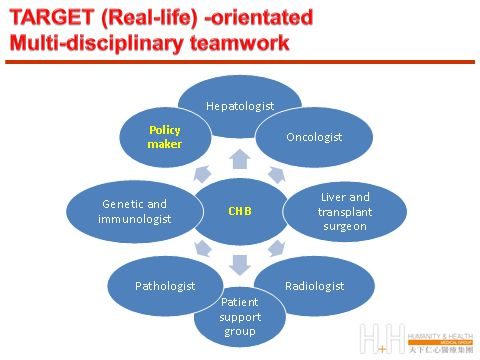
只有多學科共同努力,並在社區和監管部門的幫助下,清楚認識真正的「治療終點」,我們才能減輕慢性乙型肝炎患者的痛苦。
參考文獻:
1. B.S. Blumberg, H.J. Alter, S. Visnich, JAMA 191 (1965) 541–546.
2. Beasley RP, et al. Lancet 1981,2(8256):1129-1133
3. Chen DS. Science. 1993 Oct 15;262(5132):369-70
4. Chang MH, et al. N Engl J Med. 1997, 26;336(26):1855-9.
5. Liaw YF, et al, N Engl J Med. 2004, 7;351(15):1521-31.
6. Lok AS, et al. Hepatology. 2016 Jan;63(1):284-306.
7. Jiang Y, et al. Nature, research letter, https://doi.org/10.1038/s41586-019-0987-8
專家簡介


廖家傑(George Lau)
中國香港內科醫學院院士,中國香港大學內科醫學博士,英國皇家內科醫學院院士,中國香港大學內外全科醫醫學士,中國香港醫學專科醫學院院士(腸胃及肝臟科),愛丁堡皇家內科醫學院榮授院士,倫敦大學皇家內科醫學院榮授院士
中國香港天下仁心醫療集團主席,中國香港天下仁心胃腸及肝臟中心主任,中國人民解放軍總醫院第五醫療中心聯合中國香港天下仁心醫療集團C肝診斷治療中心共同主任,中國人民解放軍總醫院第五醫療中心聯合肝病轉化醫學研究所共同所長
Decades of research in chronic hepatitis B infection-Have we reached the endpoint?
Being a practicing hepatologist in Asia for almost thirty years, my most distressing clinical experience is the need to confront patients with chronic hepatitis B (CHB) infection, being undiagnosed and presented with advanced/late hepatocellular carcinoma (HCC). Many of these patients subsequently died at their middle-age.
With the discovery of hepatitis B surface antigen (as Australian antigen), by Nobel Prize winner Baruch S. Blumberg in 1963 coupled with subsequent large scale epidemiology study (Beasley RP 1981, Chen DS, Sung JL 1977) and the demonstration of substantial reductions of HCC with universal hepatitis B vaccination (Chang MW 1997) the causal relationship of CHB with HCC, has been clearly established.?
In addition, CHB infection is also the major culprit of end-staged liver cirrhosis and its reactivation, either spontaneously or with the use of immunosuppressive therapy could lead to fulminant liver failure. Hence, from the clinical perspective, the ultimate endpoint for the treatment of CHB should be a reduction of its related morbidity and mortality i.e. HCC, end-staged liver cirrhosis and fulminant hepatic failure due to its reactivation.
With the understanding of viral life cycle and the importance of host immunity, two interferon (IFN, standard and pegylated) and seven nucleos(t)ide analogues: lamivudine, telbivudine, entecavir, clevudine (in South Korea), adefovir, tenofovir and tenofovir alafenamide, have been approved by various regulatory authority for the treatment of CHB.
These medications were approved based on well-designed randomized controlled trials (RCTs), using surrogate treatment endpoints, such as e-seroconversion, reduction of serum HBV DNA level, loss of hepatitis B surface antigen (HBsAg) with or without development of hepatitis B surface antibody (anti-HBs) and improvement of liver stiffness, which are believed to be 「equivalent」 to reduction of HBV-related liver morbidity and mortality.
In keeping with this, long-term carefully designed and controlled studies with the use of lamivudine has been shown to be effective in reducing liver-related mortality and morbidity (Liaw 2004).
However, in real-life over the past two decades, HBV-related morbidity and mortality has not been reduced as expected. In a recent systemic review, which included 73 studies, of which 59 (15 RCTs and 44 observational studies) reported clinical outcomes, only moderate-quality evidence supported the effectiveness of antiviral therapy in CHB patients in reducing the risk of cirrhosis, decompensated liver disease and HCC.
By 2018, liver cancer (at least 80% being HCC) remains the fourth leading cause of cancer death worldwide, with about 841,000 new cases and 782,000 deaths annually (mortality/incidence ratio of 0.93). Annually, over 50% of all newly diagnosed liver cancer cases (> 90 % related to HBV) and deaths occurred in China. On the other hand, due to the increase use of immunosuppressive agents for treatment of various forms of cancer, immune-related diseases and in patients who received transplantation, there is an increasing incidence of fulminant liver failure due to HBV reactivation.
What is the missing link between science and real life? Recently, major research effort has been focused to achieve loss of HBsAg with or without development of anti-HBs and eradication of intrahepatic cccDNA as a form of 「cure」 in CHB. This is supported by the previous observation that in CHB patients, loss of HBsAg before the development of liver cirrhosis will drastically reduce the incidence of HCC.
Will this type of 「cure」 be a better solution in terms of efficacy, safety and cost (with the widespread utility of much cheaper generic agents) as compared to life-long nucleos(t)ide or finite therapy with IFN (though only a minority could have sustained disease remission), to achieve the ultimate endpoints with elimination of HBV-related liver morbidity and mortality?
What we really need is perhaps methods for early detection of HCC (to allow curative local ablation therapy or transplantation) based on the new technology platform in genomics and proteinomics, raise of public and health-care professional awareness of CHB, designation of more effective algorithm to further reduce incidence of new infection and to understand and to correct the other comorbidity factors which can aggravate CHB-related morbidity and mortality.
Only with conjoint multi-disciplinary effort, with the support of our community and governance agency, with clear understanding of the real 「end-point」, will we be able to alleviate the suffering of mankind from CHB infection.